
Wesley Chiang
Wesley serves as one of the team leads and guides the material selection and design validation of the product. He also has background in medical device startups and directs the company's business model.
All current crutches on the market meet some, but not all, of the five design criteria necessary for a successful crutch. YCrutch has designed a crutch that meet all five listed below.
Current crutches that have improved the functionality and comfort of crutches have also vastly increased the cost. These cruthces are not affordable for the common user. YCrutch promises an improved design within a reasonable price range for its consumers.
The common crutch employed by clinics and hospitals are not meant for long-term use. For this reason, many users of it experience adverse side effects such bruising and soreness. The YCrutch is designed to prevent such discomfort.
All crutches on the market have limited adjustable features. This means that the crutch does not adequately fit the specific limb conformations and needs of each user. The YCrutch provides more seamless and a larger range of adjustments for the forearm lengths and heights of each user.
A concern for redesigned crutches are their ability to maintain the support of the user. The YCrutch has been tested to support weights beyond 300lbs.
The conventional crutch is hard to walk with. It provides strong support while standing, but it is not easy to walk with. The YCrutch is designed to better mimic a natural motion during use to allow the user to feel as if they are walking normally.
Annually, 21 million US citizens suffer from lower limb injuries that require the use of the crutch. Of such cases, 1 in 5 (around 4 million), experience adverse medical conditions due to the inadequate design of the outdated crutch used in hospitals and clinics. Such medical conditions range from severe bruising to pinched nerves and soreness from extended bad posture. The yCrutch serves to eliminate all such problems from the crutch.

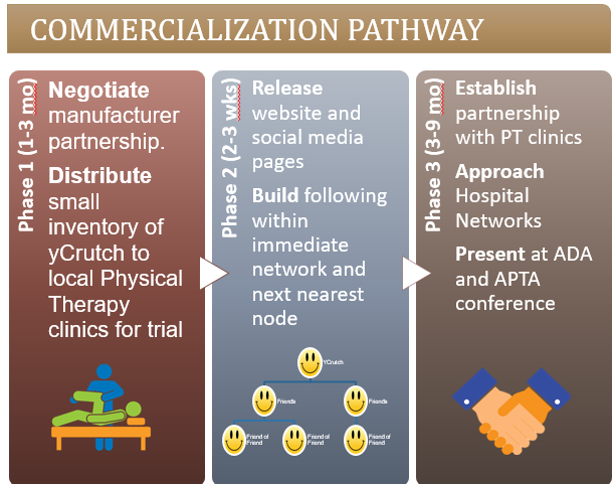
YCrutch plans to reach commercialization through a 3-phase approach. The pathway begins with establishing strong credibility with small, local clinics that have the ability to give detailed patient feedback on the yCrutch. Then, through a social media marketing strategy, yCrutch will develop a strong public interest and backing that will help it penetrate into the larger market with hospital networks and disability associations. The yCrutch projects to achieve net sales of $100k by the start of Phase 3 (12 months) and spike to $1 million by a year after Phase 3 (24-27 months).
The yCrutch product is classified in the FDA Class II category and qualifies for 510(k) premarket notificaiton exemption through Part 878 "General Hospital and Personal Use Devices." Thus, the only regulation preventin further marketing and commercialization of yCrutch is the protection of its intellectual property. YCrutch is currently in the patenting process, thus further info on the design is not available for public disclosure.

YCrutch is led by 5 University of California, Irvine engineers specializing in biomedical and materials science. The team is currently advised by Kyle Bulloch from ThermoFisher.

Wesley serves as one of the team leads and guides the material selection and design validation of the product. He also has background in medical device startups and directs the company's business model.

Brandon serves as the other team lead. He specializes in CAD modeling and machining. He designs and assembles the YCrutch prototypes.

Samantha manages purchasing for the team and designs all marketing and presentation pieces.

Shyen helps verify the prototype designs to ensure they meet design criteria. He also communicates with industry advisors for our team.

Cyrus has a previous degree and work experience in accounting. He advises the team's finances.
ycrutch.uci@gmail.com